Baxter Announces CE Mark of New EVO IQ Infusion System for the United Kingdom and Ireland
Delivers a state-of-the-art system with Dose IQ Safety Software to help elevate the standard of patient safety
User-friendly infusion system built to deliver intuitive clinical workflows and help optimise clinician efficiency
Exclusive integration with One Set System to help save nursing time and reduce opportunities for contamination1
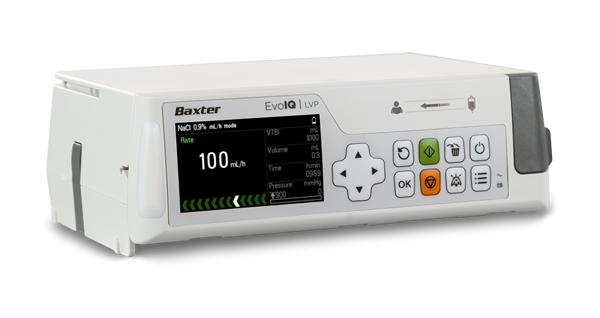
Evo IQ Infusion System
COMPTON, Berkshire, UK -
Baxter Healthcare Ltd, a leader in innovative technology for medication delivery, has announced the CE marking of the Evo IQ Infusion System in the UK and Ireland, along with the recent regulatory approval in Australia. This represents the first in a series of planned regulatory submissions for the Evo IQ Infusion System in countries around the world, with commercial launch activities starting in the third quarter of 2018.
The Evo IQ Infusion System features a scalable platform and user-centric design that includes an advanced drug library and dose error reduction software to promote patient safety; intuitive clinical workflows to help optimise clinician efficiency; and Baxter's One Set technology, which allows clinicians to easily switch between gravity and pump applications without changing sets, reducing disconnects and set changes and may help reduce opportunities for touch contamination, along with helping to reduce IV tubing use and costs.
"With the Evo IQ Infusion System, Baxter is extending its leading-edge infusion systems technology to help increase drug library compliance and protect patient infusions in the UK and Ireland," said Andy Goldney, General Manager, UK, Ireland and Nordic, Baxter. "The Evo IQ Infusion System provides hospitals with access to Baxter's infusion safety technology in an adaptable system that can grow with their needs."
Designed to Promote Patient Safety through Drug Library Compliance
Drug libraries are the defining feature of smart pumps, designed to help clinicians identify infusion pump programming errors. The drug library allows hospitals to curate a list of medications and fluids and set medically appropriate dose ranges. If programming goes beyond the limits specified for that medication, smart infusion pumps—like the Evo IQ Infusion System—will alert clinicians before they administer the medication and in some cases, prevent the delivery of the medication.
Every Evo IQ Infusion System works with Baxter's customisable Dose IQ safety software to streamline programming steps and help reduce the chance of error and is designed to facilitate effective use of drug libraries, including:
- Simplifying and expediting the hospital pharmacy’s drug library creation with support from a Baxter specialist
- Automatically defaulting to the drug library when powered on and requiring fewer programming steps to start an infusion
- Providing dosing feedback at the point of use, including drug name and both soft and hard safety limits
- Enabling easy drug library updates to help ensure programming is based on the most up-to-date parameters
Engineered to Provide a High Standard of Efficiency
At launch, the Evo IQ Infusion System will include a large volumetric infusion pump, with a pipeline of complementary technology to launch in the future, including additional pump and set offerings that will enable healthcare facilities to expand and upgrade the Evo IQ platform. Evo IQ Infusion System features designed to help increase hospital efficiency include:
- A modular and upgradable design, with features that make training faster and can potentially reduce implementation, training and servicing costs
- Lightweight and portable pumps with stacking and docking station options that are easy to move, clean, maintain and set up
- Clear organisation of critical infusion parameters on screen
- Prioritised alarms differentiated by severity to help reduce alarm fatigue
For more information on the Evo IQ Infusion System, please visit www.EvoIQInfusion.com.
About Baxter
Every day, millions of patients and caregivers rely on Baxter's leading portfolio of critical care, nutrition, renal, hospital and surgical products. For more than 85 years, we've been operating at the critical intersection where innovations that save and sustain lives meet the healthcare providers that make it happen. With products, technologies and therapies available in more than 100 countries, Baxter's employees worldwide are now building upon the company's rich heritage of medical breakthroughs to advance the next generation of transformative healthcare innovations.
For safe and proper use of this device, refer to the full Instructions for Use.
This release includes forward-looking statements concerning Baxter’s Evo IQ Infusion System and Dose IQ Safety Software, including potential benefits associated with their use. The statements are based on assumptions about many important factors, including the following, which could cause actual results to differ materially from those in the forward-looking statements: satisfaction of regulatory and other requirements; actions of regulatory bodies and other governmental authorities; product quality, manufacturing or supply, or patient safety issues; changes in law and regulations; and other risks identified in Baxter's most recent filing on Form 10-K and other SEC filings, all of which are available on Baxter's website. Baxter does not undertake to update its forward-looking statements.
Baxter, Evo IQ and Dose IQ are registered trademarks of Baxter International Inc.
1Optimal frequency of changing intravenous administration sets: Is it safe to prolong use beyond 72 hours?, Issam Raad, MD; Hend A. Hanna, MD. MPH; Aneer Awad, MD; Amin Alrahwan, MD; Carol Bivins, RN; Asma Khan, MD; Deborah Richardson, RN; Jan L, Umphrey, MPH; Estella Whimbey, MD; Georganne Mansour, RN. March 2001